We are a group of healthcare professionals dedicated to fighting diseases together with our community. We are a CLIA Certified, CAP Accredited, California State Licensed Clinical Laboratory founded in 2017 and located in San Jose, California. Since November 2020, we have provided clinical tests for infectious disease (including COVID-19 tests) to over 100,000 clients from all over the country. Apostle Diagnostics is a member of California Coronavirus Testing Task Force. We were also 1 of the 2 designated COVID-19 testing sites endorsed by the San Francisco Chinese Consulate General. Our lab is co-directed by Wenqi Zeng, MD, PhD, FACMG and Charles Strom, MD, PhD, FAAP, FACMG, HCLD. Our lab personnel are professionally trained specialists in the field of life sciences and clinical diagnostics.
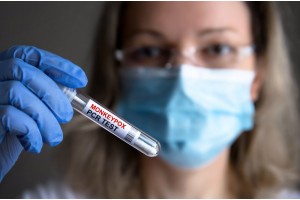
In response to the recent Monkeypox breakout, we offer fast same-day turnaround Monkeypox rtPCR tests to our community, to help fight this disease together.
Apostle Diagnostics Clinical Laboratory is listed as FDA notified Monkeypox Molecular testing lab. Notifications as described in Section IV.A.2 of the guidance – Monkeypox Diagnostic Tests Developed and Performed by Single-Site High Complexity CLIA-Certified Laboratories. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/monkeypox-and-medical-devices#Laboratories